Corroded metal parts and products are something that most people have had experience with, most of the time in an unpleasant way. The corrosion of metal exposed to the elements must be treated carefully, as corroded parts will lose structural integrity and look unappealing.
Aluminum, and its corresponding alloys, have become some of the most commonly used metals in a long list of industries. Their corrosion resistance is a big part of that popularity, particularly when compared to alternatives such as steel.
Are you considering using aluminum for an upcoming project and interested in the corrosive-related characteristics of the metal? If so, this article will provide you with a helpful overview.
Table of Contents
What is Corrosion?

Corrosion is the gradual destruction of materials when exposed to certain environmental conditions. This process results from an electrochemical reaction between one or more materials and an electrolyte.
Although it can affect other materials, corrosion is most commonly seen in metals. The most widely known metal corrosion is rust, the orange, flaky oxidation film that forms on iron and alloys containing iron. Technically, other forms of corrosive oxidation on metals such as copper and aluminum are not “rust.”
There are many types of corrosion that fit into two categories:
- General corrosion, which affects a large area more or less uniformly, and
- Localized corrosion, which is most aggressive in small, concentrated areas.
These two categories of corrosion can be further subdivided into other forms of corrosion, which can be exacerbated or slowed by factors such as temperature, electrolyte chemistry, or even the presence of other metals.
How Does Corrosion Affect Aluminum and Its Alloys?
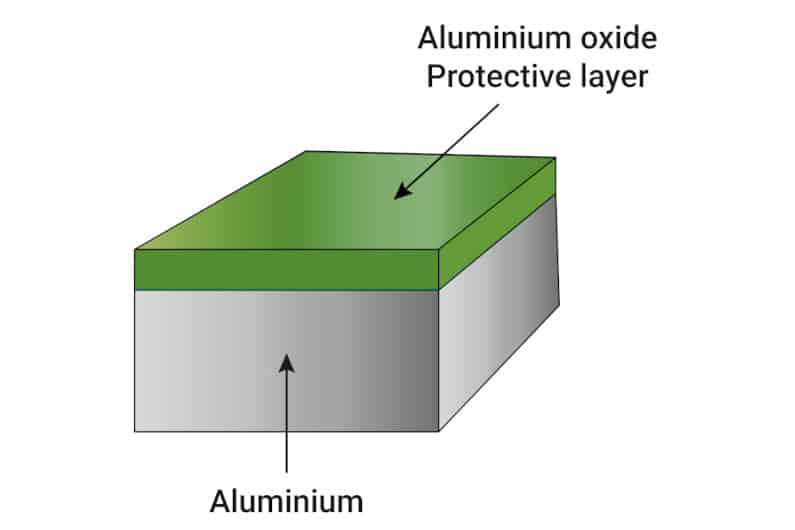
In its pure form, aluminum also oxidizes when in contact with water or humid air, just like iron. However, unlike iron, the layer of oxide most commonly formed by aluminum is not flaky or overly porous. It serves as a shield preventing any further oxidation of the aluminum.
In a way, aluminum creates its own defense against corrosion by forming a layer of aluminum oxide on its surface.
This natural protection of aluminum works slightly differently depending on the aluminum alloy in question. Aluminum is often alloyed with other materials, such as magnesium, copper, silicon, and zinc, to attain specific properties.
These alloying elements can enhance the aluminum, for example, by increasing its strength, workability, wear resistance, or ductility. However, they also diminish aluminum’s natural corrosion resistance. In particular, alloys of the 2XXX series (alloyed with copper) and 7XXX series (alloyed with zinc and small amounts of magnesium) have much lower corrosion resistance when compared with pure aluminum.
The Impact of the Environment on Corrosion Resistance

The conditions to which aluminum alloys are exposed can also heavily impact their corrosion resistance. The acidity or basicity of the surrounding environment of aluminum will substantially affect on its corrosion resistance.
A Pourbaix diagram of aluminum, which plots the zones of electrochemical equilibrium for aluminum depending on the pH level of the environment, shows how susceptible to corrosion it becomes when the pH levels become too acidic or too basic.
Aluminum is also in particular danger when used in an environment where galvanic corrosion can occur, which is when two different metals are electrically connected. This is because aluminum tends to be the less noble, or more reactive, metal in these situations. Under galvanic corrosion situations where aluminum is at risk, it will corrode at an accelerated rate instead of the other metal.
For this reason, aluminum anodes are commonly used as a cathodic protection measure for other metals, using the aluminum anode as a sacrificial element that breaks down over time to prevent corrosion on the other metal, often made of steel.
Lastly, situations that allow crevices and pits to contain a buildup of harmful chemicals may lead to localized corrosion in aluminum parts and equipment. The affected areas will have hard, brittle, white deposits, which can compromise the integrity of the aluminum for certain applications, causing leaks in aluminum storage tanks, for example.
Can You Enhance Aluminum’s Corrosion Resistance?

There are some ways in which you can enhance, or supplement, aluminum’s natural corrosion resistance:
• Anodizing is a process that thickens the naturally occurring protective oxide layer by submerging the metal in an electrolytic bath (seen above). This boosts the protective capabilities of the oxide layer and permits color dyeing of the surface to produce a very aesthetically pleasing finish.
• Alodining is a type of conversion coating for aluminum parts that creates a thin protective film on the metal. Although not quite as effective as anodizing, it prevents corrosion and can be used as a primer for subsequent painting.
• Painting an aluminum surface with a high electrical resistance liquid paint can prevent galvanic corrosion by preventing contact between the aluminum and other metals. This is also a finishing process that can offer an almost unlimited amount of color options and can be used in small batches of products.
• Powder coating is another type of finish that can prevent galvanic corrosion, just like liquid paint. In this case, however, the powder bonds to the aluminum, which reduces peeling and chipping behavior. The color availability is also extensive, and it’s more environmentally friendly than liquid painting options.
Consider Alloys, Environment, and Finishing Carefully

When considering aluminum for a project, you’ll always have to be careful about the nuances of the corrosion resistance this material offers. Although in its pure form aluminum will be very corrosion-resistant, its alloys may not be as exceptional at preventing corrosion.
That being said, alloys such as 5052, 5083, 6063, and 6082 all offer an impressive corrosion resistance under most circumstances. Just be sure to consider your specific environmental and operating conditions when thinking about using aluminum in a situation where corrosion could be a concern.
If corrosion is a concern, consider enhancing corrosion resistance by applying one of the surface finishes mentioned above to protect your product.
To learn more about the types of aluminum surface finishes available, see the article below.

![Pourbaix diagram of aluminium [22].](https://www.researchgate.net/profile/Michaela-Fousova/publication/331722518/figure/fig2/AS:746417383542784@1554971180131/Pourbaix-diagram-of-aluminium-22.png)



